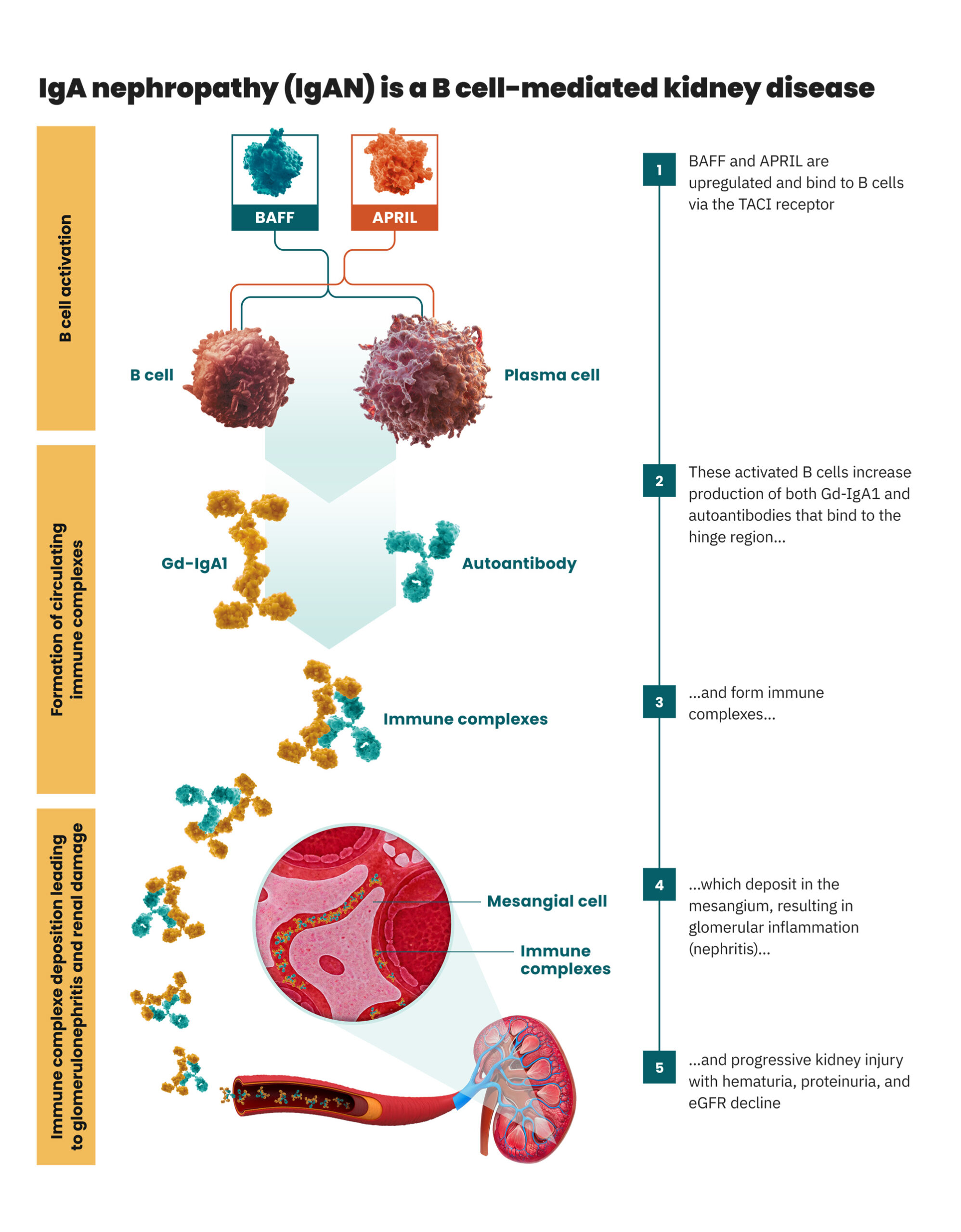
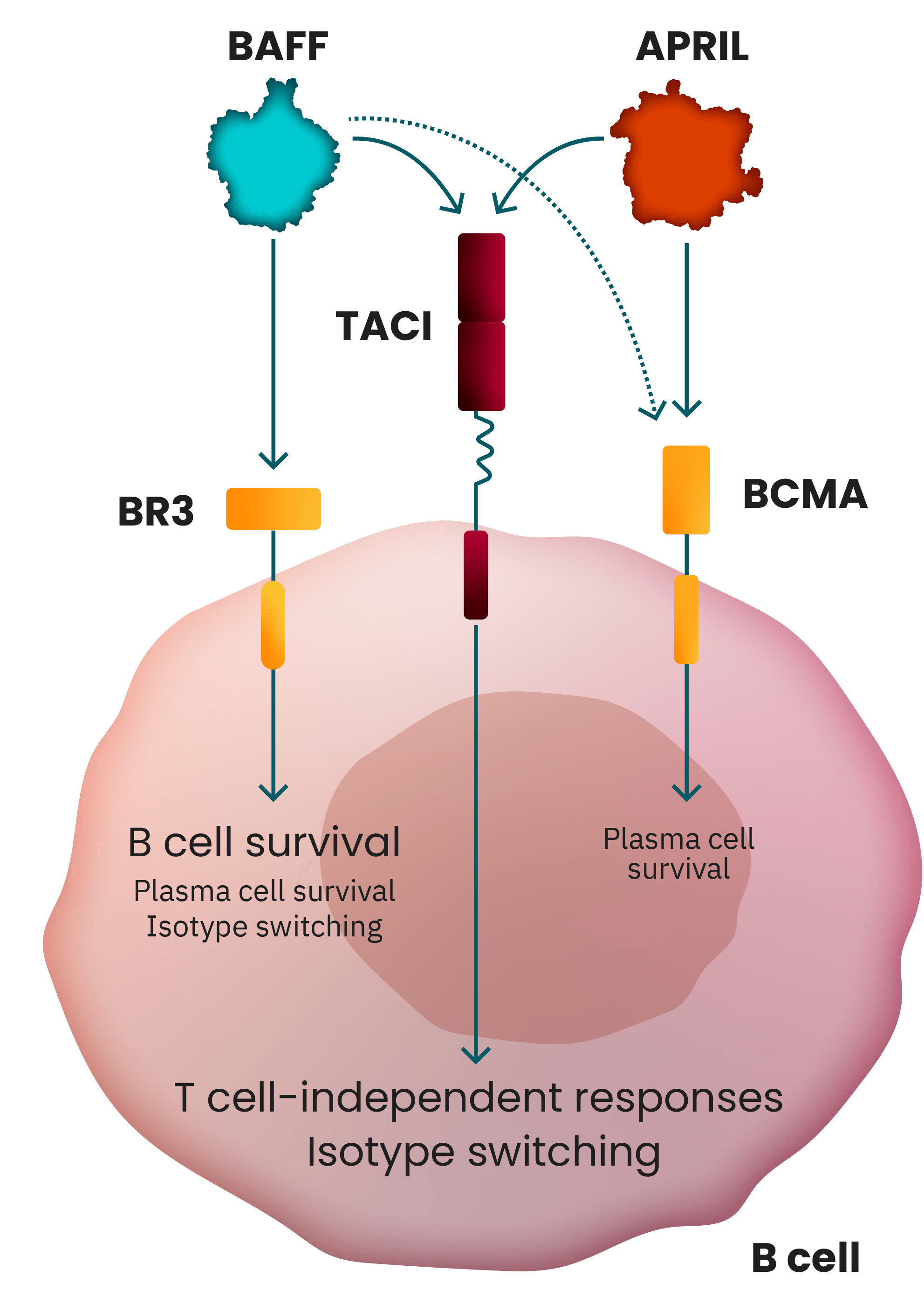
Both BAFF and APRIL play a crucial upstream role in
regulating B cell function in IgA nephropathy (IgAN)
pathogenesis.1
B cell production of both pathogenic Gd-IgA1 and autoantibodies, which form immune complexes, is the
root cause of IgAN. The cytokines B cell activating factor (BAFF) and a proliferation-inducing ligand
(APRIL) modulate B cell activity via the transmembrane activator and calcium modulatory and cyclophilin
ligand interactor (TACI). When upregulated BAFF and APRIL bind to TACI, B cells are stimulated to
increase the production of Gd-IgA1 and autoantibodies, activating a cascade of events that leads to
progressive kidney damage in IgAN.1
An overview of IgAN pathogenesis1–3