Hematuria is a common feature of IgAN, and may be associated with worse clinical outcomes13
Hematuria is a common clinical manifestation of IgAN, with more than 70% of patients developing microscopic hematuria.13
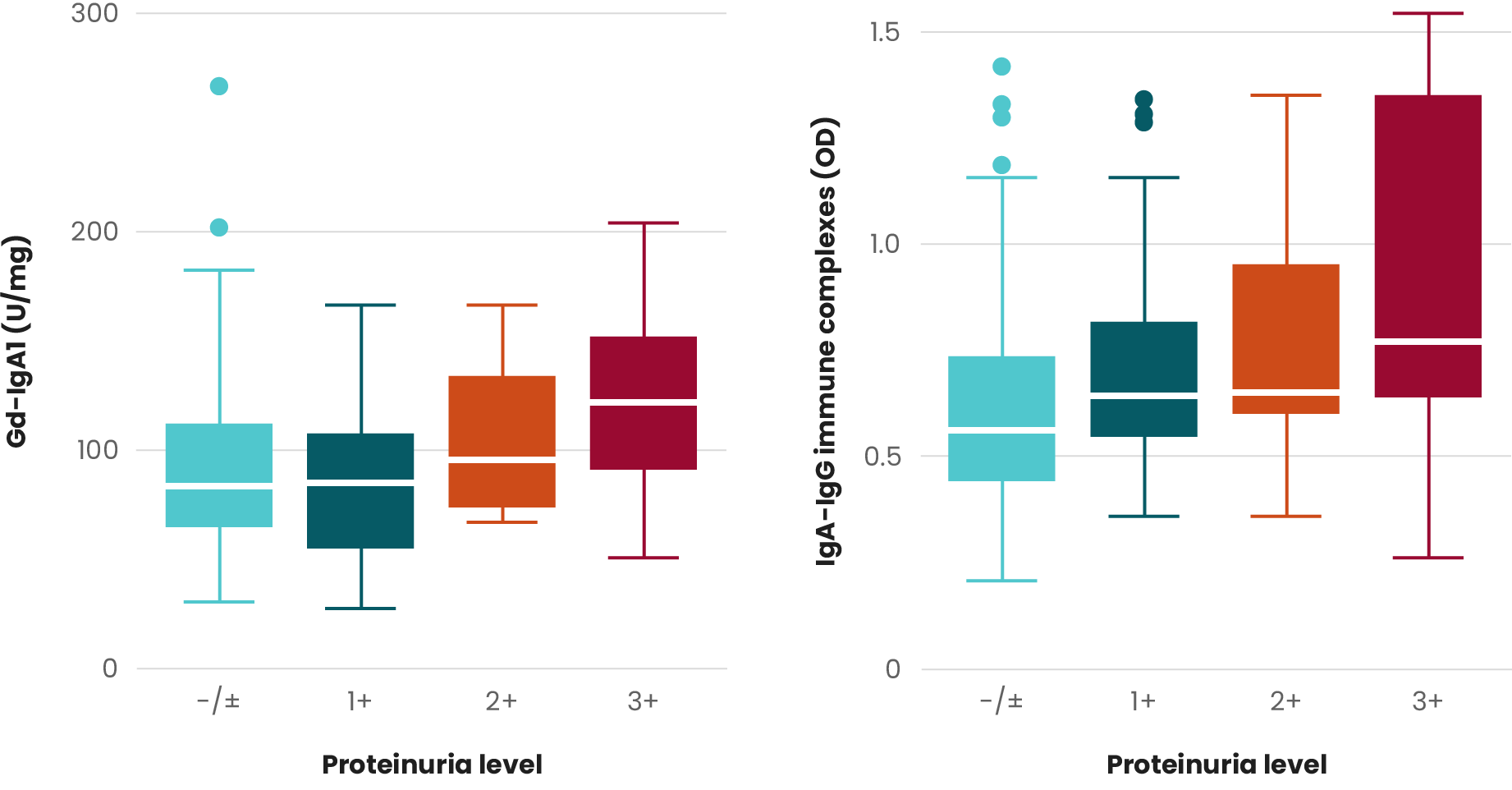
In IgAN, the deposition of immune complexes in the kidney induces the release of cytokines, chemokines, and complement activation, leading to inflammation. Local inflammation, in turn, causes glomerular capillary damage—which allows the passage of red blood cells into the urine—and the development of hematuria.8
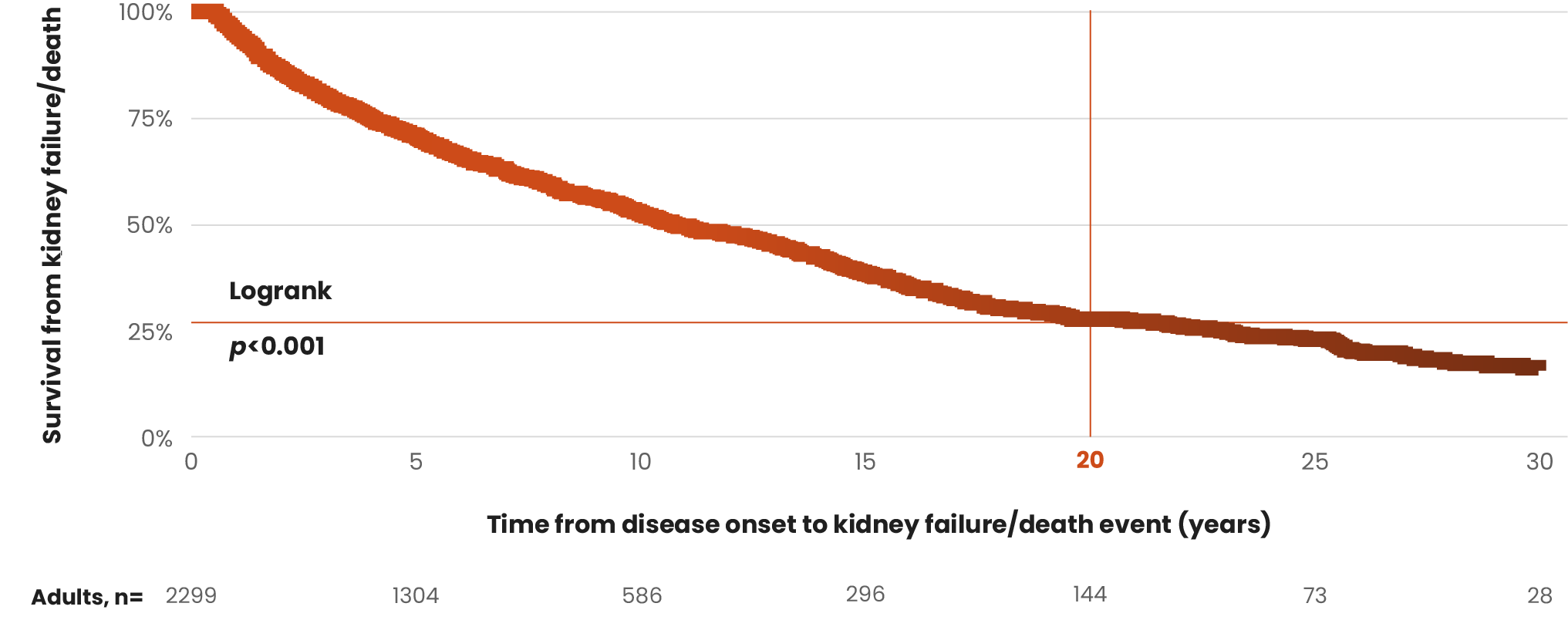
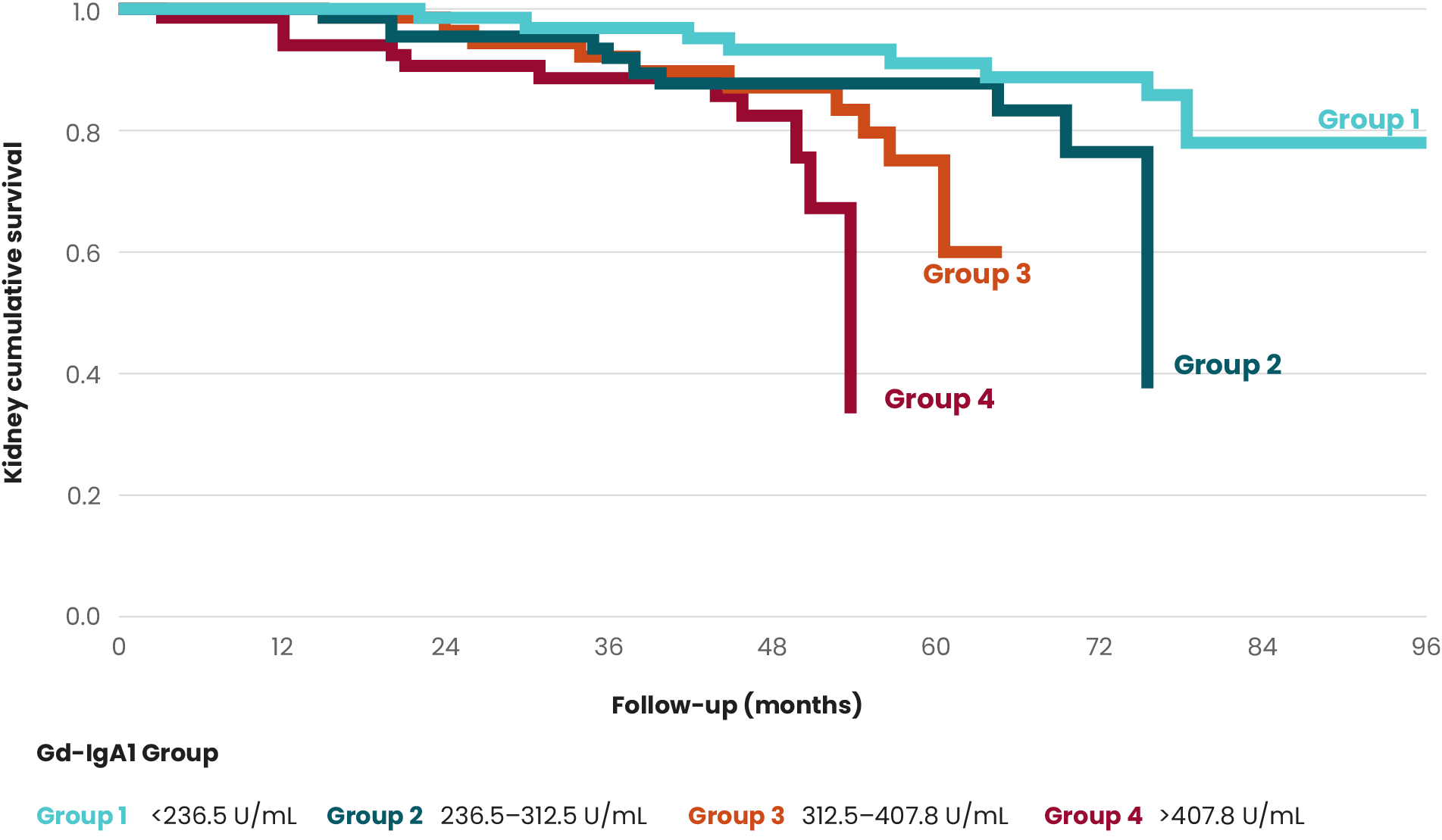
Both the presence and persistence over time of hematuria are associated with higher rates of kidney function loss and kidney failure.14
Persistent microscopic hematuria was associated with a significant 87% increase in the risk of kidney failure in the long term.15
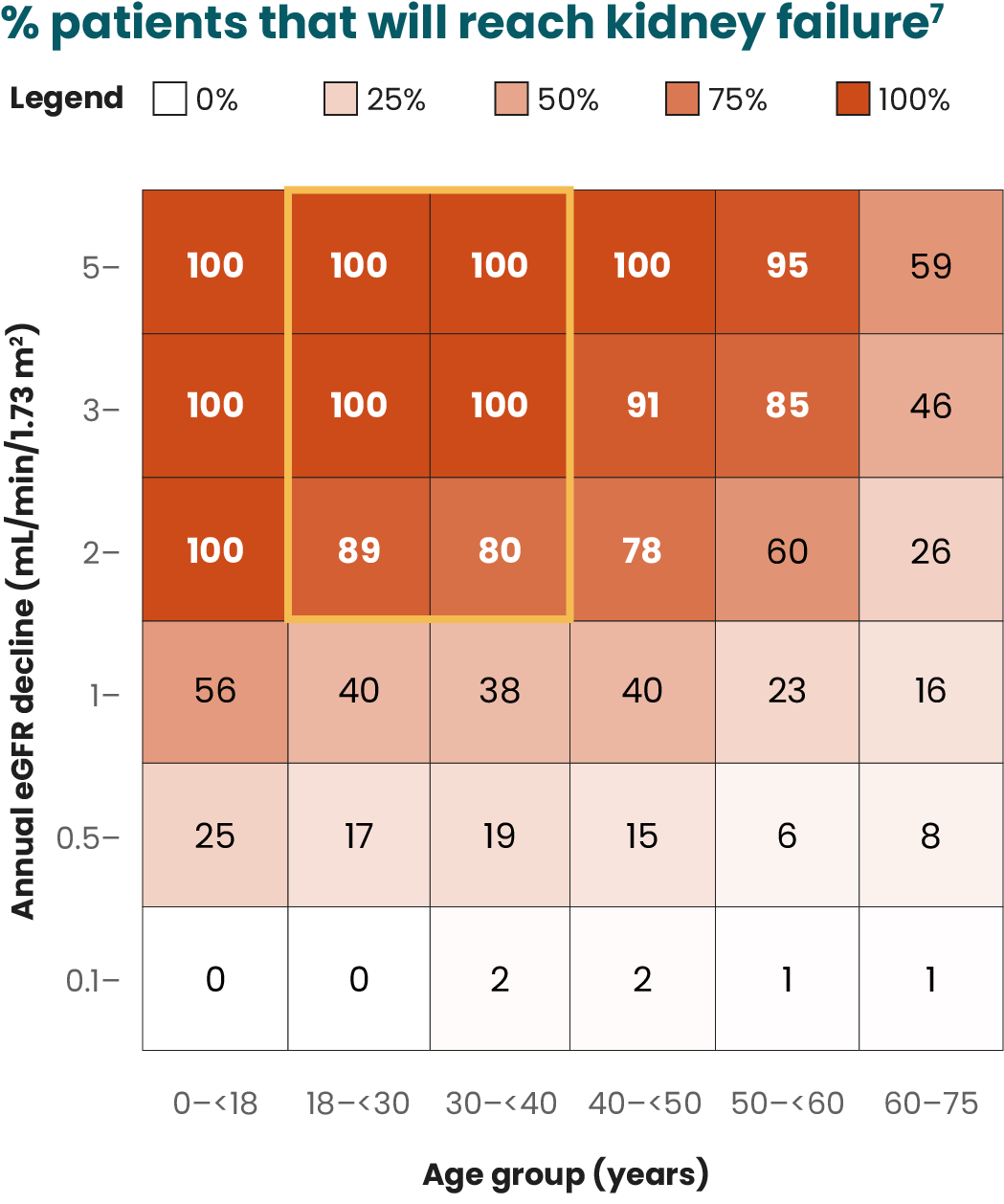
Although the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines single out proteinuria as an independent risk factor for IgAN progression, several studies suggest hematuria as a potential predictor of progression to ESKD.2,12,13,15
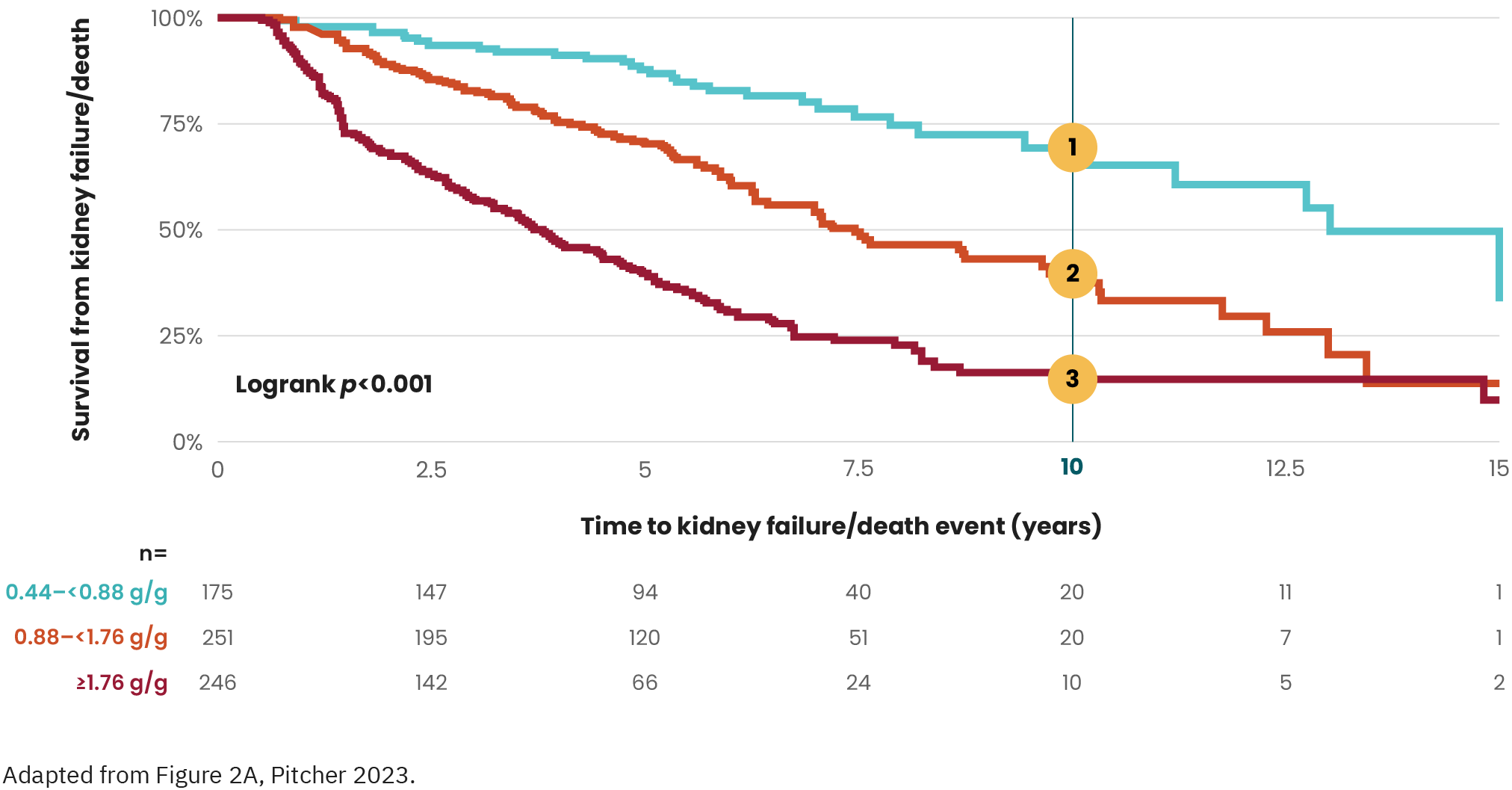
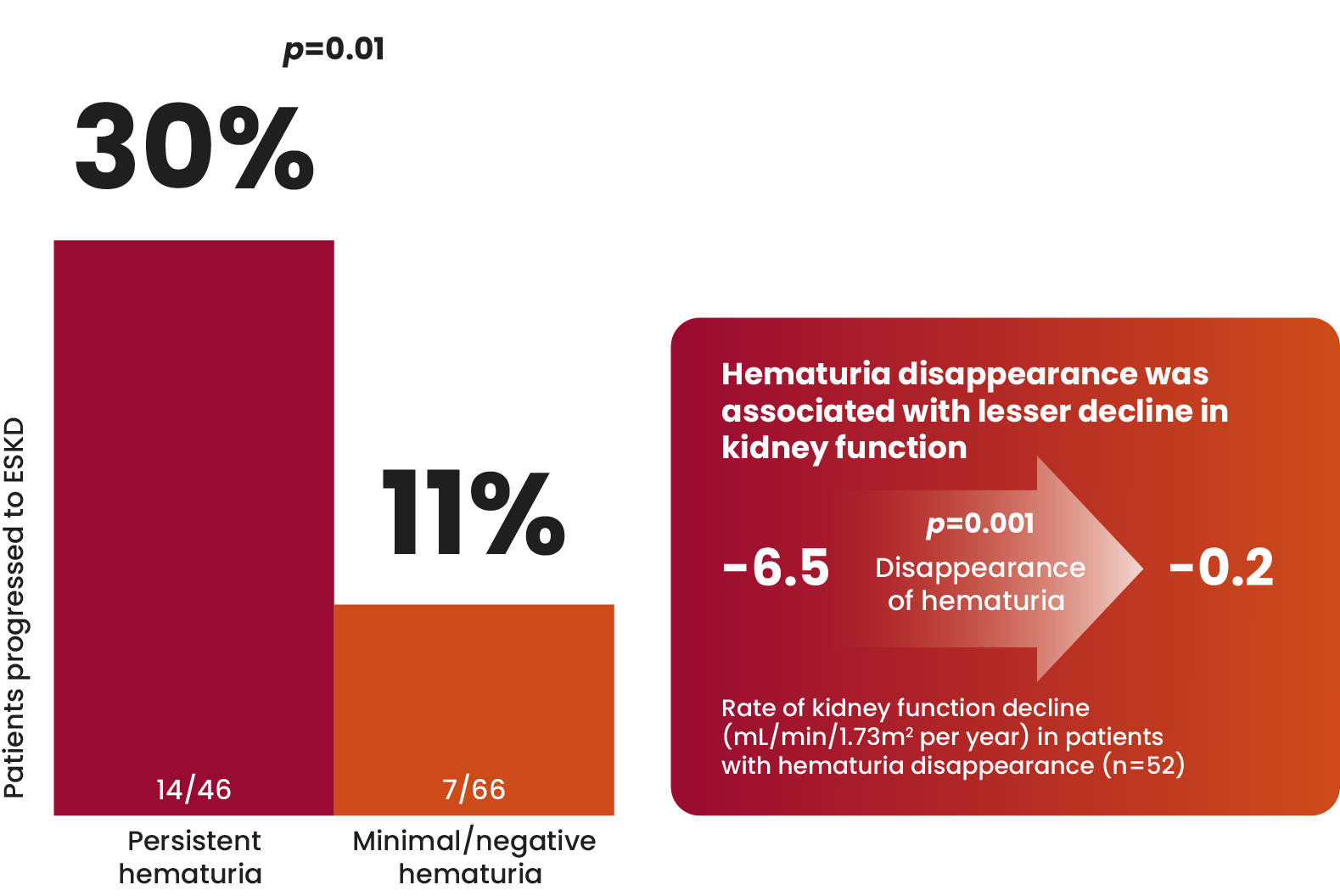
Long-term cohort study of patients with IgAN (n=112)
Both time-averaged hematuria and proteinuria were independent
predictive factors for ESKD.14
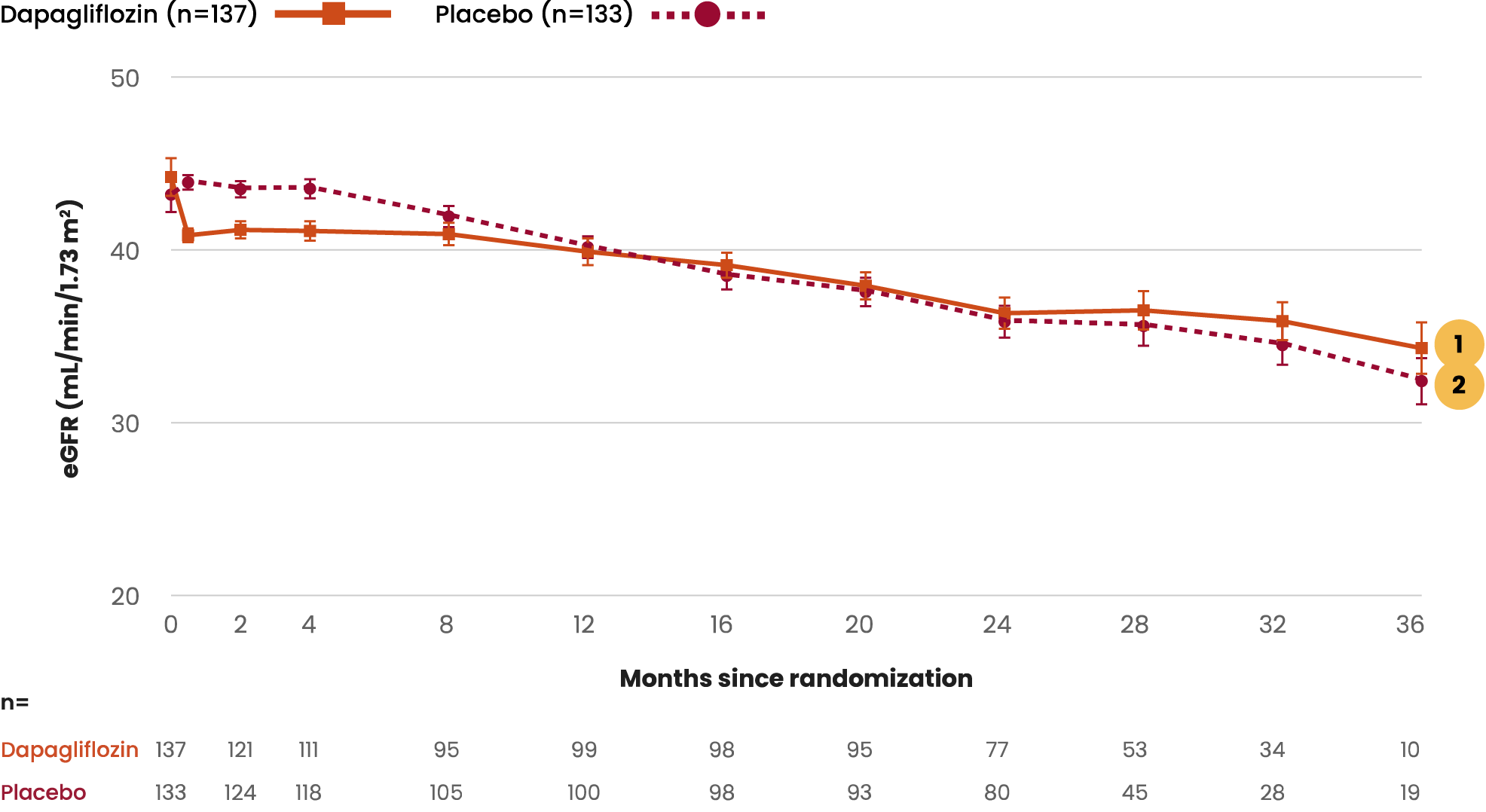
Resolution of microscopic hematuria has been associated with
improved kidney outcomes in patients with IgAN.14